Abstract
Introduction:
Lumbar punctures (LP) are routinely used to administer intrathecal chemotherapy for children and adults with hematologic malignancies. The platelet threshold at which an LP can be safely performed is a heavily debated topic. A platelet count of ≥50x10 9/L is a widely accepted threshold for other image-guided procedures [1]. In this patient population achieving this platelet count can be challenging. Previous studies failed to report any increase in the incidence of post-procedure adverse events in patients with a platelet count below this threshold who undergo LPs [2,3,4]. However, these prior studies were limited in their sample size for the number of LPs done in patients with platelets <50 x 10 9/L.
Objective:
Our study aims to identify differences in adverse events related to the administration of intrathecal chemotherapy in patients with platelet counts <50 x10 9/Lcompared to those over the transfusion threshold of ≥50x10 9/L.
Methods:
A retrospective chart review of all patients who received intrathecal chemotherapy under fluoroscopy guidance between January 2020 and May 2021 at Moffitt Cancer Center was performed. Patient charts were reviewed to collect information on baseline characteristics, laboratory parameters, platelet transfusions, and post-procedure outcomes. Adverse events related to LPs or platelet transfusions were compared among patients above or below the platelet threshold of 50x10 9/L. Statistical analysis was performed using chi-square test.
Results:
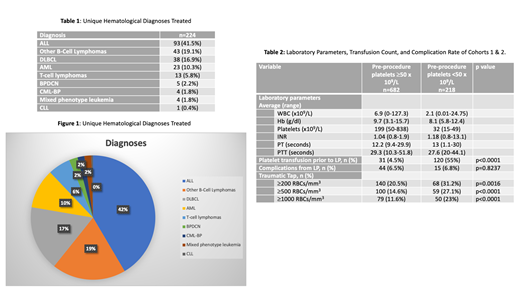
A total of 900 LPs performed on 224 unique patients were included in the study. The median age at LP was 60 years (range 20-87). 107 (47.7%) patients were females and 117 (52.3%) were males. 169 (75.5%) patients were Caucasian, 22 (9.8%) African American, 3 (1.3%) Asian, 3 (1.3%) Hispanic, and 27 (12.1%) were unknown. The hematological malignancies for which intrathecal chemotherapy was administered included: acute lymphoblastic leukemia, diffuse large B cell lymphoma, other B-Cell Lymphomas, acute myeloid leukemia, T-cell lymphomas, blast-phase chronic myeloid leukemia, mixed phenotype acute leukemia, and chronic lymphocytic leukemia (Table 1, Figure 1).
The 900 LPs were divided into two cohorts based on the pre-procedure platelet count: Cohort 1 included 682 LPs (75.8%) with a pre-procedure platelet count ≥50x10 9/L, and cohort 2 included 218 LPs (24.2%) with a pre-procedure platelet count <50x10 9/L. The average laboratory parameters for the cohorts are presented in Table 2. The average platelet counts for cohort 1 and cohort 2 were 199 (range 50-838) and 32 (range 15-49), respectively. Patients with a pre-procedure platelet count of <50x10 9/L were transfused at a significantly higher rate prior to the procedure (55% vs 4.5%, p<0.0001). Only one patient experienced an adverse reaction from platelet transfusion in the form of allergic reaction with facial swelling, treated and resolved with diphenhydramine. A total of 59 post-procedure adverse events were reported but were not significantly different between the two groups (6.5% vs 6.8%, p=0.8237). No instances of epidural hematomas were reported. The most common complications included headaches (n=42), back pain or soreness (n=11) and nausea or vomiting (n=6). Only two patients experienced bleeding complications, including subarachnoid hemorrhage and subdural hemorrhage, but neither of these was directly linked to the procedure. However, the rate of traumatic taps was significantly higher in the group with platelets <50x10 9/L with observed LP RBC count ≥200 (31.2% vs 20.5%, p=0.0016), ≥500 (27.1% vs 14.6%, p<0.0001), and ≥1000 (23% vs 11.6%, p<0.0001).
Conclusion:
Among 900 LP procedures, a pre-procedure platelet count <50x10 9/L did not demonstrate a higher rate of hemorrhagic complications (6.5% vs 6.8%, p=0.8237) but did demonstrate a higher rate of traumatic taps. No instances of epidural hematomas were seen. However, the number of pre-procedure platelet transfusions required was higher in the group with lower platelets. In light of this, consideration should be given to lowering the platelet threshold for performing LPs. While prospective studies are necessary to confirm that a lower platelet threshold is safe, a lower platelet threshold would result in decreased burden on blood banks by reducing platelet transfusions and could potentially aid in decreasing platelet alloimmunization and subsequent sequelae.
Jaglal: Novartis: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal